
Capital Raising
CLOSED
Neopharma Technologies Ltd
The Worlds Leading Drugs of Abuse and Health Data Solution
INDUSTRY
Medical
Investment Highlights
Company Overview
Products & Services
Transaction Overview
Additional Information
Team
News

Neopharma Technologies Ltd
This offer closed August 2024. If you would like to discuss anything in relation to this company please contact us for further information.
All information on this page was current as at the date of closure.
Investment Highlights
US FDA Cleared and CLIA WAIVED NEOTEST Drugs of Abuse Testing Products
CE Approved NEOTEST Drugs of Abuse Testing Products
NEOVAULT ISO 27001, HIPPA and GDPR certified
myNEO and NEOVAULT apps cleared on the Google Play and Apple Store in the US
IT Development of leading integrated AI Vision technology with Test Device recognition and Test Strip Recognition and Reporting
Company Overview
Neopharma Technologies Ltd is an Australian public unlisted company that has wholly owned subsidiaries in Malaysia and the USA and has developed a range of industry leading rapid tests and participates in the current $35 Billion (USD) rapid diagnostics market growing to $134 billion (USD) by 2030.
Neopharma aims to be a global leader in the diagnostics market with 16 industry leading and technologically AI integrated products covering Drug Testing, Infectious Disease and Pregnancy. Anchored by its global patented TamperLoks medical device and US trademarked myNEO and NEOVAULT technologies.
All Neopharma products are supported with a fully integrated IT Test Data management platform NEOVAULT, and smartphone apps myNEO and NEOVAULT for the secure collection and recording of test data.
Neopharma will officially launch its recently secured US FDA cleared NEOTEST products and its NEOVAULT ISO 27001, HIPPA and GDPR certified enterprise resource management platform at the SAPAA (Substance Abuse Program Administrators Association) conference in the USA in October 2023.
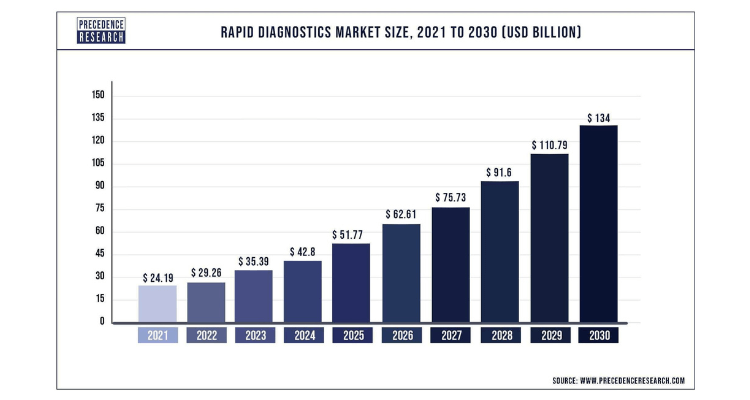
WHY Neopharma
- Recently received US FDA certification and CE approval for its NEOTEST range.
- Significant product pipeline of approvals to further open up the Australian, European and South East Asian markets with extensive product range.
- A low cost large scale business model.
- Blue sky opportunity to reach $1B + valuation with NEO’s patented technologies and approved test kits paired with NEOVAULT.
- Achieved highest international security and compliance standards with ISO 27001.
- Pipeline of planned audits to achieve GDPR, SOC 2 and HIPPA certifications with a pathway for FedRAMP certification within 6 months.
- myNEOand NEOVAULT app’s approved and now available on the google play and apple stores ready for commercialization.
- NEOVAULT has AI and AR integrated technologies. With ability to sell big data to insurance companies and federal government.
- Australian based Company Headquarters with wholly owned subsidary’sin Malaysia and US ready to scale.
- Significant IP with Global Utility and Design Patents and Registered Trademarks. Neopharma Technologies SbdBhdis ISO 13485 certified.
- After completing company Due Diligence research, Australian Wholesale Investor Market platforms, Onmarket & Venture Crowd have assisted the company raise over $1.5m from professional and sophisticated investors.
Products & Services


NEOTEST & myNEO: the World’s first Covid-19 testing app
Designed by Neopharma Technologies used with all US FDA and TGA-approved Covid-19 antigen and combined Covid-19 antigen & Flu AB test kits for the US and Australian markets.

Drugs-Of-Abuse Testing – Tamperloks Datavault
TamperLoks enables a rapid drugs-of-abuse urine test and assigns a unique QR code to the candidate being tested.
TamperLoks DataVault is a global leader in drugs-of-abuse testing. There is no current comparable hardware and technology pairing in the market globally.

Tamperloks – Rapid Drug Test Device
Patented 3-chamber tamperproof drugs-of-abuse urine testing device.
Test results within 90 seconds.
Patents ensure asset protection with further expansion into new markets.

General Disclaimer
The content on this site is provided solely for information purposes, is not a recommendation or an offer to buy or sell a security and is not warranted to be correct, complete or accurate. This site is intended for Accredited/Sophisticated/Wholesale investors only. By continuing with an investment, you are self-accrediting that you so qualify. To the extent permitted by law, neither PrimaryMarkets, its affiliates, nor the content providers (such as the issuers of securities who appear on the site) are responsible for any investment decisions, damages or losses resulting from, or related to, the content, data and analyses or their use. The investment opportunities on this site and any statements made about them by their issuers are not vetted or verified by PrimaryMarkets. Investing in securities in private or unlisted companies and Funds is speculative and involves a high degree of risk. The investor must be prepared to withstand a total loss of their investment. We strongly encourage the investor to seek independent financial, professional or investment advice before investing in securities. The presence of an investment opportunities on this site should not be interpreted as an implied endorsement of it by PrimaryMarkets. Some content provided may constitute a summary or extract of another document. Past performance does not necessarily indicate future performance. The content on this site was current as at date of initial publication but may not be current as at the date of your viewing. For a more complete understanding of all the terms and conditions of your use of this site click here.
Want to learn more?
Fill in the expression of interest form below



